Introduction:
The year 2023 will mark the culmination of a decade-long journey known as US DSCSA, a mandate aimed at securing the drug supply chain. This journey commenced with the serialization of pharmaceutical drug units and has since evolved beyond the mere generation of unique serial numbers. It now encompasses an identifier within the serialization cycle, triggering checkpoints as pharmaceutical units traverse the supply chain. The requirements of the Drug Supply Chain Security Act (DSCSA) are generally applicable to all transactions involving finished prescription drugs for human use, including the trading partners engaged in these transactions.
Key Requirements of US DSCSA 2023:
- Facilitating electronic data exchange with both direct and indirect trading partners.
- Verification of suspect and illegitimate products.
- Processing misalignment exceptions.
- Implementing credentialing and authentication for trade partners.
For those who joined the journey later, here's a brief recap of the US DSCSA's progression:
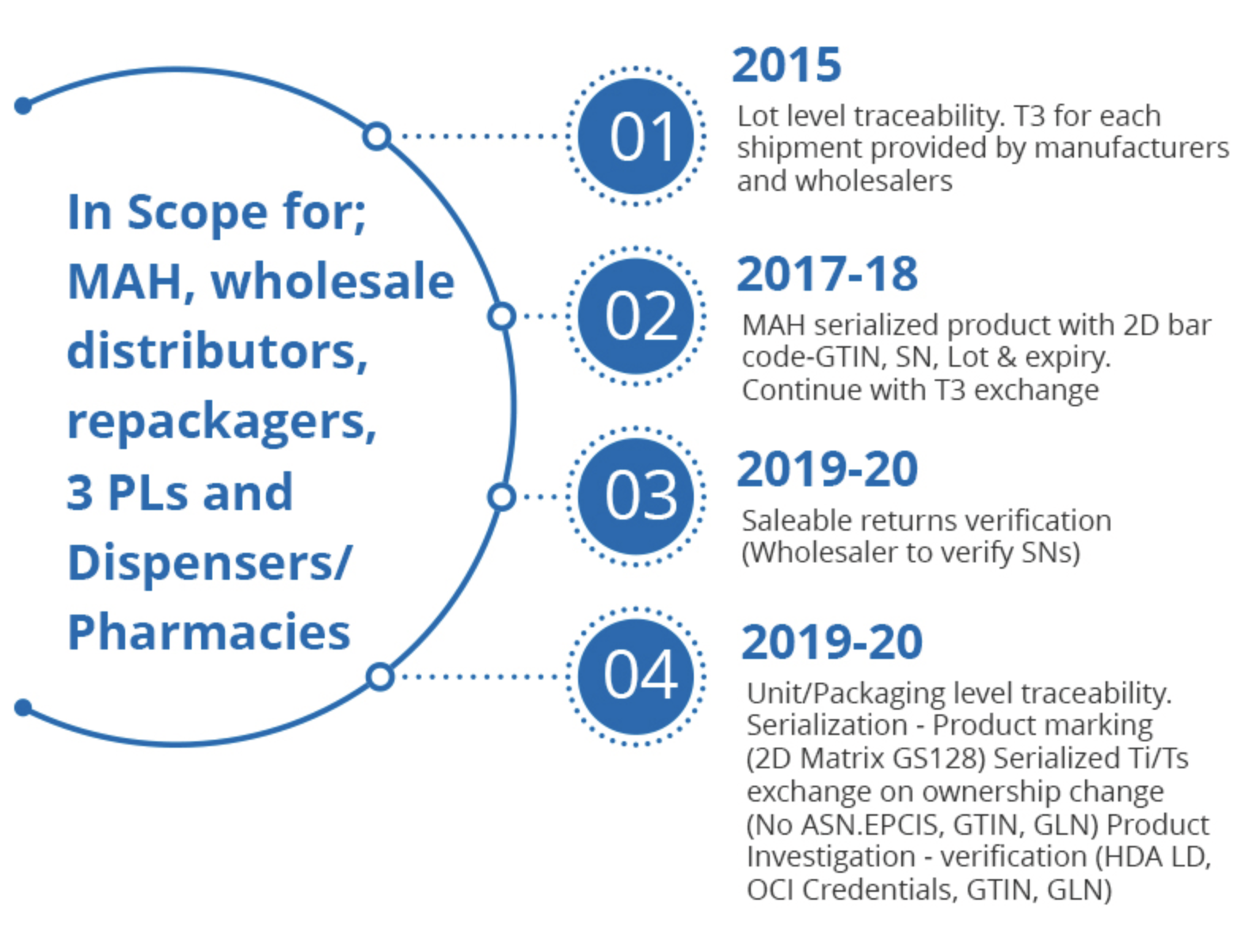
An area of concern for supply chain entities is identifying, comprehending, and resolving misalignment exceptions. These exceptions arise when a disparity occurs between the physical supply chain and the virtual supply chain. They pertain to supply chain scenarios where US DSCSA-required transaction information (TI) doesn't fully align with the physical products being transacted.
Approximately 8 to 10 billion package-level transactions occur annually, making errors and discrepancies unavoidable. The process for handling exceptions must remain separate from that of investigating suspect products. An exception might trigger a suspect product investigation, but not necessarily so.
Participants: Manufacturers, wholesale distributors, and re-packagers must establish systems and processes to detect and mitigate misalignment exceptions related to the sale of physical products. Dispensers should have systems and processes to either:
- Identify misalignment exceptions, or
- Reconcile each physically saleable unit received with the corresponding TI.
When a misalignment exception is identified, the concerned package(s) should not undergo further transactions until the exception is comprehended and resolved. Trading partners must possess systems and processes to detect, understand, and address misalignment exceptions.
Exception Scenarios: The following business scenarios may lead to misalignment or exceptions:
- Product, No Data
- Inadequate TI data: Receiving extra packages beyond the ordered quantity, yet the TI data reflects the ordered SN quantity.
- Absence of TI data: Receiving packages as ordered, but without accompanying TI data.
- Partial TI data: Receiving packages as ordered, but with TI data reflecting fewer or incorrect serial numbers.
- Data, No Product
- Insufficient product quantity: Receiving fewer packages than ordered, while TI data indicates additional serial numbers.
- Lack of product packages: Receiving TI data for the ordered quantity, but no corresponding product packages.
- Partial product quantity: Receiving TI data for the ordered quantity, but only obtaining a partial quantity.
- Data Issues:
- Receiving the complete product package quantity and TI data, but missing the respective GTIN in the buyer's master data.
- Discrepancies in the barcode, where the lot number in the TI data doesn't match the encoded lot number.
Overall Takeaway in Exception Management:
- A well-planned receiving process aids in the early identification of exceptions during distribution, facilitating timely resolution.
- Collaborating with relevant stakeholders expedites exception resolution.
- Understanding supply chain business use cases assists in identifying ownership changes and potential exceptions.
- Adopting industry best practices to standardize data flow interoperability can reduce misalignment exception occurrences.
How can CosmoTrace help?
We provide serialization consulting, implementation, and integration services to help our clients manage end-to-end serialization projects and prepare them for the existing and upcoming regulations across the globe.
We are well versed with the compliance regulations for various markets and can help you to implement the serialization requirements.
Our team of experts strategizes and plans end-to-end solutions using a combination of years of knowledge in product serialization, pharmaceutical supply chains, life sciences, and brand integrity.
Disclaimer:
This information is being provided ‘As Is’ with no claims of suitability for a particular purpose. It represents just one possible interpretation of information available in the public domain or through membership organizations, and that interpretation is subject to change. This information does not constitute legal advice. Users must refer to the source material for the complete requirements and form their own interpretation before making business decisions. Please use the references below to follow the updates at the source.
Reference:


