Introduction
In 2022, the Ministry of Health (MoH) in Kuwait took a significant step towards enhancing pharmaceutical traceability by issuing a circular outlining track and trace requirements for medicinal products. While traceability is not currently mandatory, the MoH has set the stage for a transformative shift. Starting January 2024, all pharmaceutical products circulating in Kuwait must feature a GS1 Data Matrix containing four crucial data elements. Kuwait's MoH is poised to implement GS1 standards encompassing serialization, aggregation, and barcoding.
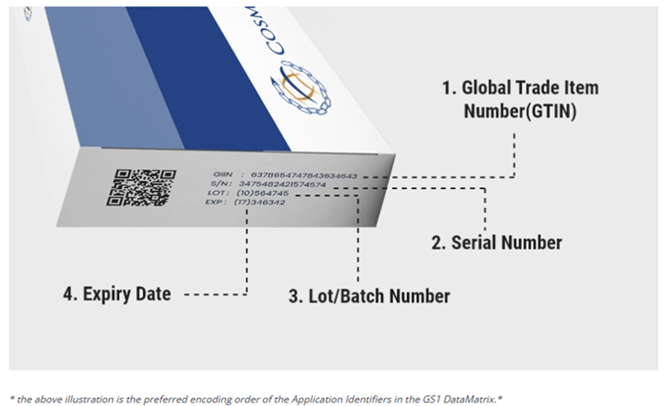
Product Identification
The 2D barcode, essential for accurate visual reading, encodes the following four data elements, complemented by Human-Readable Information (HRI):
- GTIN: N2 + N14
- Batch Number: N2 + Maximum of 20 alphanumeric characters.
- Expiration Date: Represented in YYMMDD.
- Unique Serial Number: N2 + Up to 20 alphanumeric characters.
Timelines
- January-December 2023: Local agents are required to submit master data to GS1 Kuwait.
- January 1, 2024: Every pharmaceutical product within Kuwait's supply chain must comply with the GS1 Data Matrix, featuring the four specified data elements.
Technical details on master data submission to GS1 Kuwait, reporting requirements, and additional updates on Kuwait Pharma Traceability are eagerly awaited.
Kuwait, as an active member of the Gulf Health Council (GHC), aligns with the organization's initiatives. The GHC has signaled the launch of the Electronic Patient Information Leaflet (e-PIL) project, aiming to provide updated, electronic, and harmonized information about medicines across the GCC region.
CosmoTrace's Role in Facilitating Compliance
How Can CosmoTrace Help?
CosmoTrace stands as a strategic partner, offering comprehensive serialization consulting, implementation, and integration services. Our expertise lies in guiding clients through end-to-end serialization projects, ensuring preparedness for existing and forthcoming global regulations.
Our Solutions Include:
- Compliance Expertise: Our dedicated team at CosmoTrace not only specializes in local compliance but is also well-versed in global pharmaceutical regulations. With extensive knowledge spanning various markets, we ensure that our clients seamlessly adapt to the evolving landscape of pharmaceutical traceability on an international scale. This comprehensive approach allows us to implement serialization requirements while simultaneously addressing the broader global regulatory framework. As we continue to stay informed and updated on diverse compliance standards worldwide, our clients benefit from a nuanced understanding that transcends regional boundaries.
- Consultation and Strategy: Our team of experts crafts strategic, end-to-end solutions. Leveraging extensive knowledge in product serialization, pharmaceutical supply chains, life sciences, and brand integrity, we navigate the complexities of serialization.
In a rapidly evolving pharmaceutical landscape, CosmoTrace emerges as a reliable partner, empowering clients to navigate the intricate terrain of serialization regulations and ensure seamless compliance. Stay tuned for further updates on Kuwait's Pharma Traceability as we eagerly await additional information to keep you informed.
Disclaimer:
This information is being provided ‘As Is’ with no claims of suitability for a particular purpose. It represents just one possible interpretation of information available in the public domain or through membership organizations, and that interpretation is subject to change. This information does not constitute legal advice. Users must refer to the source material for the complete requirements and form their own interpretation before making business decisions. Please use the references below to follow the updates at the source.


