Introduction to Regulation
The regulator of Indonesia’s pharmaceutical serialization practices is the ‘Indonesian Food and Drug Authority’ (BPOM – Badan Pengawas Obat dan Makanan).
Traceability started with the QR code in 2018 and gradually moved to the GS1 standards for Data Matrix codes. Adopting GS1 standards in 2D Code implementation, the Indonesian Food and Drug Authority (BPOM) requires serialization and aggregation within the scope of requirements. Serialization and traceability requirements vary according to product groups.
BPOM published the Revised Regulation on the Implementation of 2D Barcodes in Pharmaceutical and Food Traceability No. 22/2022, which will enter into force on October 5, 2022.
Key Dates for Compliance Deadlines
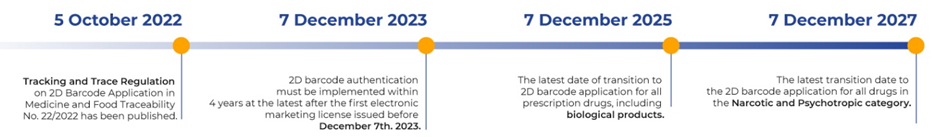
Scope of regulation: All medicines sold in Indonesia display unique 2D codes as per the GS1 standards however, the barcode implementation has two parts depending on product classification:
1. Identification Barcode (a QR barcode that identifies a product in the market and verifies the legality of a product):-
- Products that do not need to be serialized will only need an identification barcode
- Timelines: 2023
- Scope: traditional medicines, over-the-counter drugs, cosmetics, supplements, and processed foods. Manufacturers are required to acquire the barcode issued through electronic Marketing Authorisation (MA) approval.
- The code must contain the following information;
- Marketing Authorization Number
- NIE* Number.
* NIE is a license number issued by the BPOM. It consists of a combination of letters and numbers. The number of characters and length depends on the type of product. The number can be used to read whether the product was manufactured domestically or abroad, and it gives access to further product data in the Indonesian database Cek Produk BPOM.
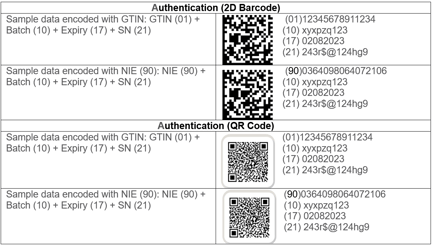
2. Authentication Barcode (a serialized product identifier and 2D barcode used in reporting and to verify a product’s authenticity by GTIN, batch (lot) number, expiry date, and serial number).-
- Products that need to be serialized will need an authentication barcode
- Timelines: 2025
- Scope: all narcotics, ethical drugs, psychotropic drugs, and other high-risk products. A business can either get it issued from the BPOM, or from an independent source.
- The authentication code must contain the following information:
- Product identification number i.e. GS1 GTIN
- Marketing Authorization Number (BPOM)
- Production code or batch number
- Expiration date
- Serial number
- Aggregation is mandatory and should follow GS1 standards.
- Not all pharmaceutical products require barcodes. Products/packs that are exempted from the requirement;
- Blister packs, strip packs, pre-filled syringes, ampoules, and tubes less than 5 mg in weight.
- Drugs having a volume of less than 5ml
- Stick packs, single packaging, suppositories, and catch covers
Conclusion:
Two barcodes will be used for serialization – Identification Barcode and Authentication Barcode. By 2023, all eligible pharma products must be marked with an Identification Barcode. By 2025, all eligible pharma products must be marked with an Authentication Barcode.
Identification (2D barcode): Sample data encoded: NIE Number (90) – (alphanumeric serial)
Reference
https://en.wikipedia.org/wiki/Indonesian_Food_and_Drug_Authority
https://jdih.pom.go.id/view/slide/5e7a1094b03f71d4de3d925146d37a0a/1423/22/2022
How can CosmoTrace help?
We provide serialization consulting, implementation & integration services to help our clients manage end-to-end serialization projects and prepare them for the existing and upcoming regulations across the globe.
Disclaimer
This information is being provided ‘As Is’ with no claims of suitability for a particular purpose. It represents just one possible interpretation of information available in the public domain or through membership organizations, and that interpretation is subject to change. This information does not constitute legal advice. Users must refer to the source material for the complete requirements and form their own interpretation before making business decisions. Please use the references below to follow the updates at the source.
.


