The UAE Ministry of Health and Prevention issued Legislative Decree No. (73) of 2021 on June 14, 2021, regarding Pharmaceutical Products Traceability. Tatmeen, the track & trace system, was introduced by UAE MOHAP to facilitate traceability of pharmaceutical units across the supply chain. Tatmeen went live on December 13, 2022.
Key areas of regulation requirements as per MOHAP:
- Article No. (3)
It is prohibited to import and trade any medicinal products within the country, or to trade any locally manufactured medicinal product, except after linking it to the Tatmeen platform.
- Article No. (4)
2D coding system is applied to all the pharmaceutical products in the country, including GTIN based on GS1 standards. GLN is to be used to monitor the change of ownership of the product.
- Article No. (5)
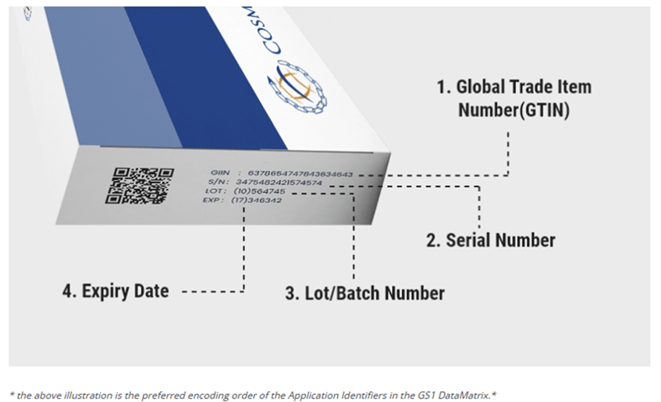
2D coding specification to include the following data elements (GS1 format);
- Global Trade Item Number (GTIN)
- Randomized Serial Number (1/10,000 randomization)
- Expiry Date
- Batch or Lot Number
Participants:
Supply Chain Partners are entities involved in producing, transporting, storing, or dispensing pharmaceutical products. They are classified as:
- Marketing Authorization Holders (MAH) who own the medicine and either manufacture it directly or use a manufacturing subcontractor. They are responsible for handling commissioning messages to Tatmeen. MAH can also have Scientific Offices in the country or the free zone.
- Licensed Agents (local distributors): Companies are allowed to import products into the country. They are responsible for obtaining an import permit from MoHAP and including it in the commissioning messages sent by MAH or them directly.
- 3PL: Companies that store and distribute medicines whether in the UAE mainland or the Free Zone. They are responsible for sending shipping messages into and out of their warehouses. While 3PLs are not currently registered with MoHAP, their master data should be present in Tatmeen; therefore, 3PLs need to register with BrandSync to get a GLN.
- Dispensaries: Pharmacies, hospitals, clinics, or rehabilitation centers that dispense products to final consumers (patients). They are responsible for dispensing messages on Tatmeen.
*Note: Some partners may have more than one role in the supply chain, and in that case, they need to handle all the messages associated with all these roles.
Prerequisite:
- Product registration in appropriate federal systems (MoHAP, BrandSync, GS1 systems).
- Entity registration as a 'supply chain partner' with an allocated GS1 GLN.
Aggregation:
- Manufacturers: Aggregate all applicable packaging levels with SSCC (up to a minimum of 2 levels of aggregation).
- Trade partners: Disaggregate and aggregate with their own SSCC up to a maximum of 1 level of aggregation.
Reporting to Tatmeen: Traceability Events (EPCIS file size 15MB or less)
- Manufacturer: report serialization, commissioning, and aggregation events.
- Trade Partners
- Importation into UAE Receipt event. Report the initial receipt of the goods into the UAE before import clearance and customs release by MoHAP and Federal customs.
- Sampling and inspecting events conducted on behalf of MoHAP during import clearance and customs release process (these events are recorded by MoHAP and/or customs inspector.
- Sampling events:
- Import clearance sampling for product verification.
- Customs release sampling for product verification.
- Quality control.
- Product transfer event to be reported by both the sending and receiving parties.
- Shipment
- Return - if the shipment is refused by the receiver. The receiver should receive the shipment and then use the return to ship back.
- Aggregation & disaggregation update event.
- Exception events.
- Destruction
- Damaged
- Stolen
Events are to be reported immediately after the physical event is completed.
Event messages that are sent to Tatmeen must be in GS1 EPCIS standards.
A single event is represented by a single GS1 event file.
The Ministry of Health and Prevention will announce the fees associated with Tatmeen at a later stage, the final decision pertaining to the fees is still pending.
Compliance
Pharmaceutical companies need to work closely with their supply chain partners and technology vendors to ensure that their serialization solutions comply with UAE MOHAP regulations and meet the required deadlines. Non-compliance with serialization requirements can result in penalties and delays in product registration and market access in the UAE.
Reference
https://tatmeen.ae/
https://www.gs1.org/
https://www.mohap.gov.ae/en/Pages/default.aspx
How can CosmoTrace help?
We provide serialization consulting, implementation & integration services to help our clients manage end-to-end serialization projects and prepare them for the existing and upcoming regulations across the globe.
We are well-versed in the compliance regulations for various markets and can help you with implementing the UAE MOHAP pharmaceutical serialization requirements.
Our team of experts strategizes and plans end-to-end solutions using a combination of years of knowledge in product serialization, pharmaceutical supply chains, life sciences, and brand integrity.
Disclaimer
This information is being provided ‘As Is’ with no claims of suitability for a particular purpose. It represents just one possible interpretation of information available in the public domain or through membership organizations, and that interpretation is subject to change. This information does not constitute legal advice. Users must refer to the source material for the complete requirements and form their interpretation before making business decisions. Please use the references below to follow the updates at the source.


