Globally the traceability areas of regulation are largely divided into 4 parts; one or more areas of regulation are applicable to the supply chain entities based on the role they play.
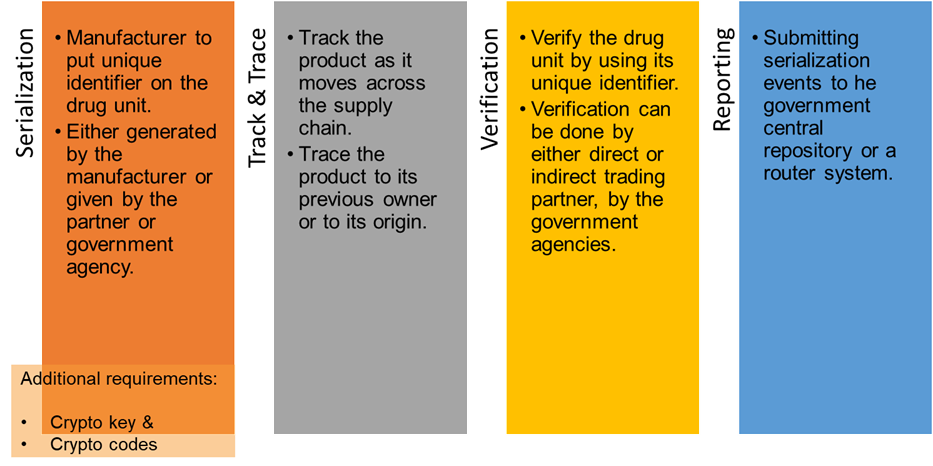
USDSCSA Roadmap :
In scope for; MAH, Wholesale distributors, and Dispensers/Pharmacies

2023: Package level traceability:
Electronic and interoperable exchange of data
- TI & TS in a secure, electronic, interoperable manner
- TI must include the product identifier at the package level
- SN, Lot number, and Expiration date will replace ASN
- NDC will be part of the product identifier
[National Drug Code (NDC) length updated from a 10-digit number to an 11-digit number in a 5-4-2 format.]
Electronic exchange of data with both direct and indirect trading partners;
- Use product identifier to verify product (expecting VRS systems to ramp up and update for 2023 requirement)
- Secure tracing of products
- Improve the efficiency of recalls
Manage alerts
- Suspected and Illegitimate product
FDA Stabilization Period - Compliance Policy Guidance
FDA is announcing two compliance policy guidelines establishing a 1-year stabilization period to accommodate the additional time that trading partners in the pharmaceutical supply chain may need to adhere to Drug Supply Chain Security Act (DSCSA) requirements for electronic drug tracing at the package level.
Under the DSCSA, trading partners – primarily manufacturers, wholesale distributors, dispensers, and repackagers – are subject to certain requirements for enhanced drug distribution security.
DSCSA 2023 requirements are scheduled to change on November 27, 2023, and will include requiring trading partners to provide, receive, and maintain documentation about products and ownership only electronically. The stabilization period will accommodate an additional year, until November 27, 2024, to allow trading partners to implement, troubleshoot, and mature their electronic interoperable systems.
Guidance 1: Enhanced Drug Distribution Security Requirements Under Section 582(g)(1).
- Use secure, interoperable, electronic approaches to exchange package-level transaction information (Ti), and exchange transaction statements (Ts).
- Have systems and processes in place to verify products at the package level.
- Have systems and processes in place to promptly respond with the Ti and Ts for a product upon a request by the Secretary, or other appropriate Federal or State official, in the event of a recall or for investigations of suspect or illegitimate product.
- Have systems and processes in place to facilitate the gathering of information needed to produce the Ti for a product going back to the manufacturer, as applicable, in the event of a request by the Secretary, or other appropriate Federal or State official, on account of a recall or for suspect or illegitimate product investigations, or in the event of a request by an authorized trading partner for purposes of investigating a suspect product or assisting with such a governmental request.
- Have systems and processes in place to accept saleable returns under appropriate conditions, i.e., being able to associate the saleable return product with the Ti and Ts associated with that product.
Guidance 2: Verification of Saleable Returns.
- To minimize possible disruptions in the distribution of certain prescription drugs in the United States, the FDA does not intend to take action before November 27, 2024, against wholesale distributors who do not verify a product identifier prior to resale or other further distribution of a package or sealed homogenous case of product as required by section 582(c)(4)(D) of the FD&C Act.
- Additionally, the FDA recognizes that some wholesale distributors may still not have systems in place by November 27, 2023. To enable the wholesale distributor to timely and efficiently comply with the verification of saleable returned product requirements, prior to November 27, 2024, the FDA does not intend to take action against a wholesale distributor for providing a transaction statement to a subsequent purchaser of product on the basis that such wholesale distributor does not yet have systems and processes in place to comply with the saleable return verification requirements.
- This compliance policy does not relieve a manufacturer of its verification obligations pursuant to section 582(b)(4)(C) of the FD&C Act upon receiving a request for verification from an authorized wholesale distributor.
United Kingdom - MHRA
The Windsor Framework sets out a long-term solution for the supply of medicines into Northern Ireland. It will ensure that medicines can be approved and licensed on a UK-wide basis by the MHRA. This will enable medicines to use the same packaging and labeling across the UK. These measures will commence on 1 January 2025. This means that after this date:
- New medicines for the UK market will be authorized by UK authorities, and packaging must carry a ‘UK only’ label to be allowed onto the UK market, including in Northern Ireland. These products will only be able to be sold in the UK.
- Medicines entering Northern Ireland will not display features required under the EU Falsified Medicines Directive (FMD) including 2D barcodes and serialization numbers that are compliant with the EU FMD Directive.
- The MHRA expects anti-tamper devices to remain on all medicine packaging.
- The MHRA will continue to allow manufacturers to supply medicines in legacy EU packaging until 31 December 2024. This extends the previous 31 December 2023 deadline requiring medicines for GB to be presented in GB-compliant packaging by the end of 2023 by one year.
- Packs in existing packaging already on the UK market, and within the supply chain, may remain until the date of their expiry.
NIMAR will continue to function to support the supply of medicines to Northern Ireland.
EU FMD Roadmap
The European Falsified Medicines Directive went live in February 2019. It impacts all EU member states and non-EU European Economic Area (EEA) members Norway, Iceland, and Liechtenstein, as well as Switzerland. The exceptions are Belgium, Italy, and Greece, which are deemed to have pre-existing features in place that will see their compliance timeline extended by a further six years.

Russia
Go-Live: 31 December 2019
Federal Law: 425-FZ
Federal IT System:
- MDLP (compliance reporting)
- CRPT (exchange of crypto codes and keys)
- Aggregation: Mandatory (for aggregation, supply chain members must report every change to individual batches; they must produce reports for each change, and report how much of the batch is left together and where there moved units went.)
- Data Elements:
- GTIN-14;
- Serial Number (13 alphanumeric characters);
- “Crypto-code”, supplied by a government service The request for a Crypto-code must include the GTIN 14 and the Serial Number data elements because the Crypto-code is specific to those data elements; – a 4-digit “key” and – a 44-character “signature”
- Lot number
- Expiry date
Eurasian Economic Union (EAEU) has proposed the use of QR codes for tracking pharmaceutical ingredients.
A new law has been drafted that proposes mandatory labeling for nutritional supplements beginning on April 1, 2023.
The pilot for nutritional supplements has been extended until February 2023, and the scope has been increased due to the reclassification and retirement of some custom codes.
Participants: MAH (Factory, Warehouse), Wholesales/Distributors, Retail and Consumers
Traceability System: ASL Belgisi (managed by CRPT Turon)
GS1 Data Matrix Standards:
- GS1 Global Trade Item Number (GTIN) (14 digit) - AI (01)
- Serial Number (13 digit) - AI (21)
- Verification key (4 digit) - AI (91)
- Verification code (44 digit) - AI (92)
Human Readable Standards:
- GS1 Global Trade Item Number (GTIN) (14 digit) - AI (01)
- Expiration Date (YYMMDD format) - AI (17)
- Serial Number (13 digit) - AI (21)
- Batch or Lot Number - AI (10)
Aggregation, a Serial Shipping Container Code (SSCC) number must be provided in a one-dimensional barcode:
- Serialization with Crypto Codes
- Aggregation Mandatory for Tertiary, Optional for Pallets
- Traceability Event Capture & Reporting
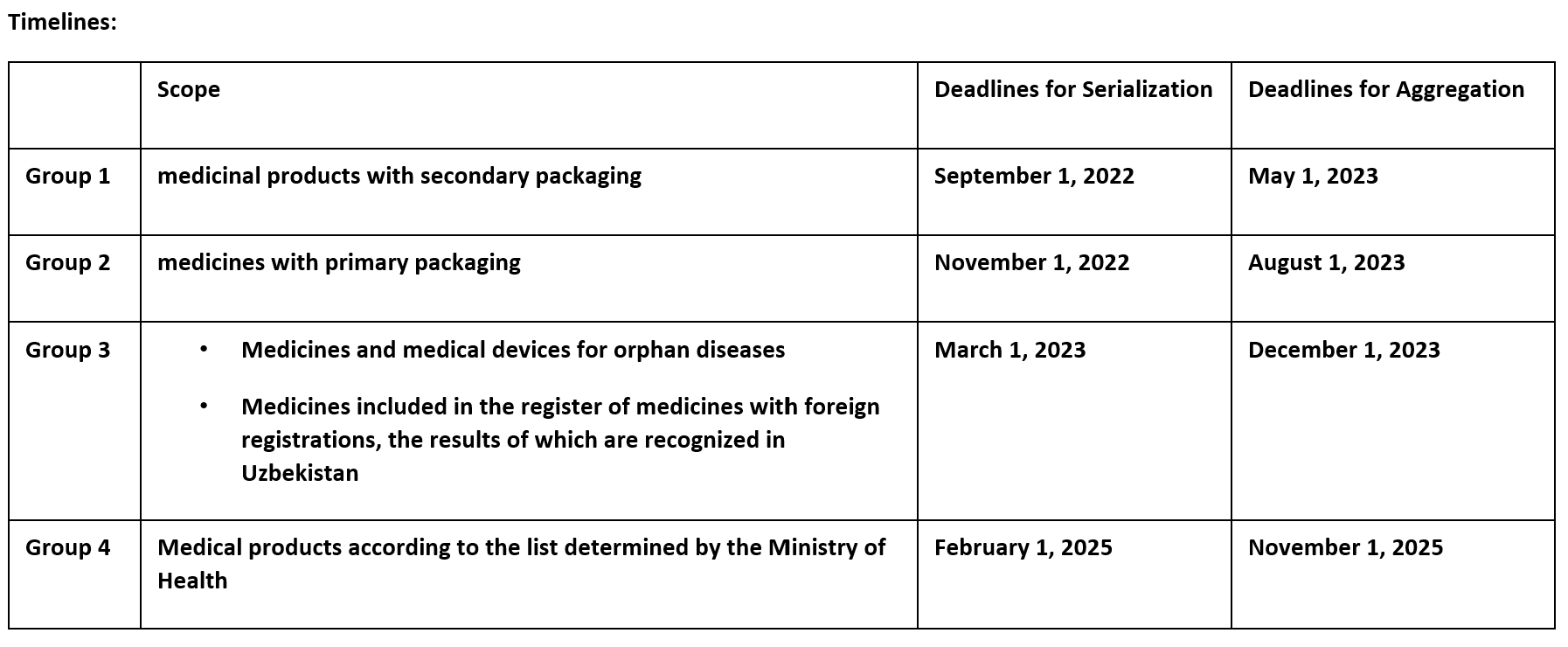
China
Go-Live: April 2019 (all drugs will be in the traceability system in 2022.)
- Aggregation: Mandatory
- Data Elements
- 2D matrix/RFID tag or a linear bar code.
- eCode: 20 digits code (7 digit product ID + 9 digit SN + 4 check digit)
- Batch
- Production date
- Pharmaceutical form
- Expiry date
To promote the construction of a drug information traceability system, the State FDA organized the formulation of two information standards;
- "Drug Traceability Code Identification Specification“(June 2023)
- "Drug Traceability Consumer Query Result Display Specification" (June 2022)
Bahrain
Implementation Roadmap
Phase 1 (MAHs) - December 31, 2021: Serialization and EPCIS reporting to MVC Hub required for Marketing Authorization Holders (MAHs)
Phase 2 (MAHs) - May 1, 2022: Aggregation to cases and pallets
Phase 3 (Customs, Agents/Distributors, Pharmacies, Dispensers) - June 1, 2022: Scanning for shipment and medicines authentication
Barcoding requirements;
- Serial Number
- GTIN
- Batch / Lot Number
- Expiry Date
Aggregation: Mandatory
Reporting: NHRA web-portal
Circular No. (05) 2023: To All Agents-Distributors (Importers) - GS1 Participation with use of NHRA-MVC Traceability Hub for Pharmaceutical Product Traceability.
As part of the Traceability initiative, a GS1-provided GLN number is required for all Agents/Distributors (Importers) in the Kingdom who are involved in the following activities:
- Making transactions inside of the DUR and/or OFOQ system relating to imports of pharmaceutical products
- Receiving transactional data from the MAH in the form of EPCIS events
A GLN can be purchased from any authorized GS1 distributor anywhere in the world for some as low as $30 as a one-off fee.
Serialization – secondary packaging
Data elements; 4 (GS1 format)
- Global Trade Item Number (GTIN)
- Randomized Serial Number (1/10,000 randomization)
- Expiry Date
- Batch or Lot Number
Aggregation: mandatory for manufacturers to aggregate all applicable packaging levels with SSCC
- Trade partners who disaggregate must aggregate with their own SSCC
- Two levels of aggregation hierarchy mandatory for UAE
Products exempted from serialization
- General Sales List (GSL products)
- Medical Devices
- Imported for personal use only
- Free Samples
- Stocks being exported from UAE FTZ
Importation of Unregistered Drugs
When the shipment import permit is created in the MoHAP Import Permit System by “Distributers/ licensed agents/ medical stores”. MoHAP Import Permit System pushes the Unregistered drugs product master data to BrandSync where additional data is entered in BrandSync. BrandSync pushes the master data to Tatmeen.
The arrival of medical products into the UAE FreeTrade Zone territory does not constitute importation until the importation is specifically initiated into the UAE market.
- The Shipping Import Permit contains the product GTIN identifiers for products that are intended to be imported.
- Each product GTIN item on the Shipping Import Permit is valid for a maximum quantity.
- The Shipping Import Permit reference must be the same reference specified in each commissioning event in the event list.
Saudi Arabia – Medical Devices Traceability Regulation
"Executive Regulation of Medical Devices Law" issued by Saudi Food and Drug Authority Board of Directors decree No. (3-29-1443) dated 19/2/1443H.
Objective: To provide standardized identification of medical devices.
Agenda: Facilitate a number of public health and safety initiatives; device traceability, identification of fraudulent medical devices, safety alerts, field safety corrective actions, incident, adverse event reporting, etc.
Scope: All medical devices and medical supplies and accessories that are intended to be placed on the KSA market and not exempted from marketing authorization requirements.
Participants: Manufacturers, Authorized Representatives (ARs)
Timelines: Implementation for unique device identification, i.e., the UDI system.
- 1st September 2022 for class B, C, and D devices > postponed to 1st September 2023
- 1st September 2023 for class A devices (low risk) > postponed to 1st September 2024
SFDA Pre-requisite:
- The manufacturer shall assign and manage UDI by following and applying the standards and specifications of one of the accepted issuing agencies (GS1, HIBCC, and ICCBBA).
- The UDI shall contain two parts: the UDI-DI and the UDI-PI(s);
- UDI-DI: unique at the product level and provides access to the information in the (Saudi-DI)* database. The UDI-DI is globally unique to a product / SKU. Below is the procedure to register the product UDI-DI;
- Sign in using the GHAD account
- Search for a product using its listing/product number and enter product DI
The UDI-DI data shall be available in the (Saudi-DI) database at the time the device is placed on the market.
- UDI-PI/s: It is unique at the unit level and includes - lot number, serial number, software identification, expiration date, or manufacturing date (mostly optional).
- The marking of the UDI does not replace any other marking or labeling requirements.
- The UDI shall be placed on the primary label of the device and on all higher levels of packaging and be presented in two forms:
- Easily readable plain-text (Human readable interpretation HRI), and
- AIDC technology (Automatic Identification and Data Capture), for example, RFID, Barcodes, etc.)
Import Control:
The importer shall submit, for each UDI-DI being imported into the KSA market
- The applicable Production Identifiers (UDI-PI/s)
- Quantity of lot-controlled devices
- Shipment date (when expected to arrive at the designated port), and
- Destination (e.g., specific distributor, hospital).
Track & Trace System (SFDA DTTS)
- All serialized medical devices will be entered into the SFDA Track and Trace system to track the device through its supply chain activities and usage in medical facilities.
- Importers and distributors shall submit and confirm their product information.
- The SAUDI-D will submit serialized medical device data into the SFDA Track and Trace system.
- The SFDA Track and Trace system will track the serialized medical device
How can CosmoTrace help?
We provide serialization consulting, implementation, and integration services to help our clients manage end-to-end serialization projects and prepare them for the existing and upcoming regulations across the globe.
Disclaimer
This information is being provided ‘As Is’ with no claims of suitability for a particular purpose. It represents just one possible interpretation of information available in the public domain or through membership organizations, and that interpretation is subject to change. This information does not constitute legal advice. Users must refer to the source material for the complete requirements and form their own interpretation before making business decisions. Please use the references below to follow the updates at the source.

