Key dates in Bahrain
November 2017 - GTINs were made compulsory to assign to all the drugs supplied in Bahrain
April 2018 -The product master data was reported to GS1 UAE BrandSync portal
December 2019 - Registration on MVC (Medical value chain) was made mandatory for all the MAHs
October 2021 – MAHs to be certified and begin sharing shipment information with the MVC Traceability Hub.
31st December 2021 – Phase 1 (Completed) - End of 90 day grace period to Invoicing Companies, MAHs and exporting distributors in submitting reports to the NHRA-MVC Traceability Hub.
Exception Period 1st October 2021 - 31st December 2021
All invoicing companies (MAHs, Manufacturers, Exporting Distributors)
- Shipments between 1st October and 31st December, 2021; shipment information during this time were reported as done in the past and NHRA exempted these shipments for reporting the serialization information.
- This was a 3-month grace period to help address present supply chain challenges which ended on 31st December, 2021
- Medicines received in the inventory during this period did not always show up in the NHRA-MVC Traceability Hub when the serialization information were scanned due to the exemption and the pandemic-related global supply chain challenges.
1st May 2022 – Phase 2 - Aggregation to become mandatory for MAHs - In progress
1st June 2022 - Phase 3 - Scanning for shipment and medicines authentication to become mandatory for Agents, Distributors, Pharmacies, Dispensers.
Introduction to NHRA
The National Health Regulatory Authority in Bahrain plays a key role in ensuring expected health standards are met. This guarantees that patients are provided with safe drugs and can access the best form of medical care. The NHRA is an independent sector that regulates the entire healthcare system in the Kingdom. It has effective regulatory procedures that involve the licensing of all parties involved in the healthcare business such as pharmaceutical centers and hospitals. The NHRA has implemented a blockchain based end to end traceability hub which is the first of its kind in the GCC.
NHRA’s role in Bahrain’s Regulatory landscape
It provides licenses for the pharmaceutical factories in order to allow them to produce and supply drugs. The manufacturers also have to go through the NHRA in order to have their new drugs registered and then be provided the mandate to sell their products. As a licensing function, the NHRA is also involved in providing licenses to healthcare professionals. As a way of acting as a voice for the patients, the NHRA also takes part in regulating the general prices of drugs and also investigating any form of misconduct done by any healthcare professional. In addition to such fundamental and necessary roles, the NHRA also accepts and declines approvals when it comes to conducting clinical trials. In order to implement the Track & Trace requirement, a national Traceability Hub “RTH” NHRA-MVC (“Medical Value Chain”) has been established. The provider and operator of the MVC Traceability Hub is Medical Value Chain W.L.L. operating through a subcontractor for Bahrain.
NHRA Bahrain’s Traceability Hub Highlights
- Meets global track and trace requirements
- Adheres to GS1 standards
- Provides end to end traceability of all medicine imports into Bahrain
- Clear visibility of medicine inventory right from manufacturers to patients and all intermediate transfers
- Provides analytics of medicine imports and usage.
- Increased patient safety by detecting and stopping counterfeit medicines.
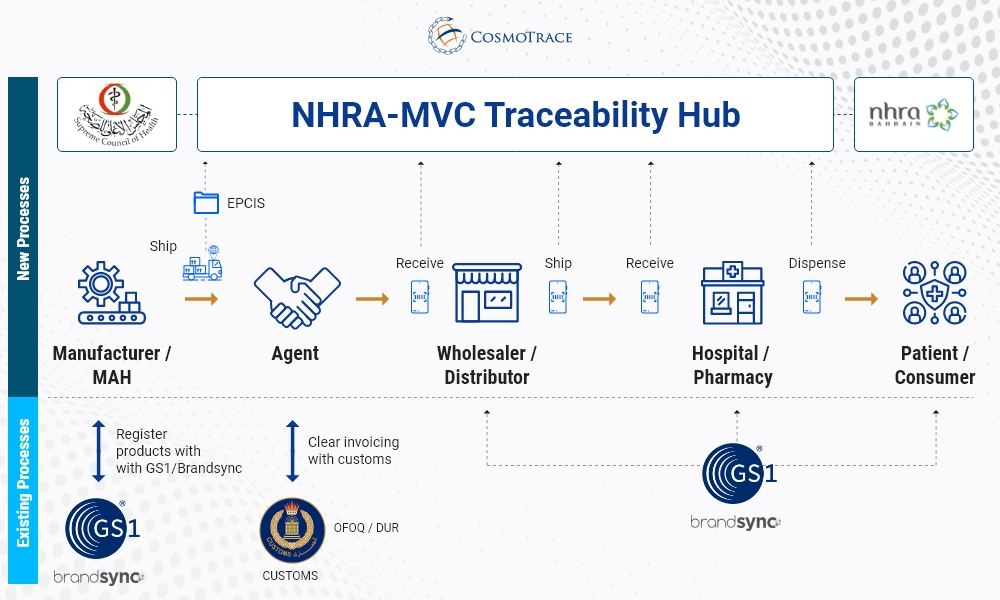
Fundamental guidelines for Traceability in Bahrain
- GS1-standard barcode to the medicines which are in-scope for Serialization, including human-readable data elements.
- Provisioning and commissioning of a unique serial number at the individual unit at the POS.
- Reporting to the Bahrain Traceability Hub – NHRA-MVC
- Recording and traceability of commissioning, aggregation, and the shipping events
- During the initial phase, Marketing Authorization Holders (MAHs) and Invoicing Companies must register and report all the medicines which fall under the scope with the NHRA.
Standards for Labeling & Barcode
The NHRA requires all pharmaceutical manufacturers to meet the GS1 defined standards for labeling the products. The following application identifiers must be encoded as per the serialization standards:
- GS1 Global Trade Item Number (GTIN) (14 digit) - AI (01)
- Expiration Date (YYMMDD is the preferred format) - AI (17)
- Serial Number (up to 20 characters) - AI (21)
- Batch or Lot Number - AI (10)
-1.jpg?width=467&name=secondary---Copy-(2)-1.jpg)
Aggregation
The NHRA requires both the case/shipper and the pallet tertiary packaging aggregation barcodes & serialization. SSCC (Serialized Shipping Container Codes) will be embedded on the tertiary label to indicate the packaging hierarchy and information of the pallet. However, aggregation is still optional in Bahrain and will become mandatory from May 2022. The following aggregation rules related to reporting and GS1 standards still apply at the time of this writing:
- Operational events in the warehouse are not needed to be reported.
- No unpacking events are expected to be reported.
- Requirement is to only report packing events
Products governed by the regulations
- Foreign-produced medicines that are imported as finished goods
- Imported medicines that are re-packaged locally
- Non-registered medicines with a temporary importation license
- Reporting responsibility lies with the invoicing company.
It’s important to note that the regulations do not make any provision for allowing 3rd parties do the reporting part with MVC Hub on behalf of an MAH. The NHRA has ensured that all parties and countries supplying and trading with pharmaceutical products meet the GS1 System of Standards.
NHRA Invoicing guidelines
All Invoicing Companies (MAHs, Manufacturers, Exporting Distributors) are required to renew their subscription fees to the NHRA-MVC Traceability Hub. The invoice can be retrieved by logging into the www.nhra-mvc.bh website.
For new Invoicing Companies (MAHs, Manufacturers, Exporting Distributors) signing up for the first time, they will be required to pay a one-time sign-up fee in addition to signing a Participation Agreement, and pay the 2022 subscription fees. They can navigate to NHRA-MVC website at www.nhra-mvc.bh to begin the first-time sign-up process.
The MAH that export products to Bahrain are required to comply with the new reporting requirements given by the kingdom. There are certain changes also if you are an invoicing company, shipping & manufacturing company or both. For more information and to check your readiness, visit our Bahrain page.
How can CosmoTrace help?
We provide serialization consulting, implementation & integration services to help our clients with managing the end-to-end serialization projects and preparing them for the existing and upcoming regulations across the globe.
We are well versed with the compliance regulations for Bahrain and help customers in establishing the automated as well as manual connection to the NHRA MVC hub. We also support customers in reporting their master data and production information to the hub by creating required documents and reports.
Our team of experts strategize and plan end-to-end solutions using a combination of years of knowledge in product serialization, pharmaceutical supply chains, life sciences and brand integrity.
Disclaimer
This information is being provided ‘As Is’ with no claims of suitability for a particular purpose. It represents just one possible interpretation of information available in the public domain or through membership organizations, partners and that interpretation is subject to change. This information does not constitute legal advice. Users must refer to the source material for the complete requirements and form their own interpretation before making business decisions. Please use the references below to follow the updates at the source.
Reference
AlSaffar, Gardenia. “Ethical Programs for Patients in Bahrain.” Ethical Consumerism and Comparative Studies Across Different Cultures: Emerging Research and Opportunities. IGI Global, 2020. 53-73.
Pascu, Georgiana Andreea, Gabriel Hancu, and Aura Rusu. “Pharmaceutical serialization, a global effort to combat counterfeit medicines.” Acta Marisiensis-Seria Medica 66.4 (2020): 132-139.
NHRA Circular No. (29) 2021
Need a helping hand in ensuring smooth operations in your serialization efforts. Contact us to get started.


