Defining the basics
Serialization is assigning a unique product identifier to the smallest saleable unit that can be uniquely verifiable.
Tracking is understanding the current and past locations of a serialized unit.
Trace is to identify the history of all entities that came into contact with the serialized unit along the supply chain.
What is the need for Pharmaceutical Serialization?
It is estimated that Pharmaceutical companies across the globe face billions of dollars worth of losses annually because of stolen or counterfeit products. One of the measures the industry took to overcome this challenge was to implement pharmaceutical serialization to protect the consumers; which in turn made it more difficult to manufacture fake goods. Serializing a product is only the first step to strengthening the supply chain. Track & Trace involves putting in place measures to identify the product and see it’s journey within the entire supply chain in order to reduce counterfeit and stolen products.
What does Pharmaceutical Serialization do?
With pharma serialization, each product is assigned a unique identifier (UID). As the laws are different in each country, the regulations are subject to change on this basis. In certain countries the entire product dose may need to be serialized while for some countries the regulations may include even the smallest commercialized or manufactured unit in its regulations depending on the prescription drug batches. Often manufacturers have set requirements to follow, unlike the requirements in place for companies that are based on the country they’re selling their products in. For example, if a company is based in United Kingdom and sells it’s products in China, Saudi Arabia and India, the regulatory requirements will be different in each country.
What is Track & Trace?
Track & Trace applies to all industries. In summary it involves tracking the journey of the product from its source to the destination within the supply chain.
The Track & Trace process is even beneficial to companies as early as from the stages of production, distribution and till it reaches the end-user. Companies enter all the necessary information (i.e. unit details, location, distributor, etc.) for the products into the systems so it’s easier to identify where they are in the supply chain. If needed, Track & Trace can easily help with the recall of products making it more cost effective to withdraw a certain compromised batch instead of an entire shipment/unit. There is no doubt that a robust system validation process needs to be in place to achieve this level of assurance and this is driven by a well executed and managed project plan for serialization.
As Track & Trace regulations vary according to each country’s laws, all products must first be serialized so they’re easily identifiable within the supply chain. Some of the additional requirements could also include reporting of the data to the respective governments and regulating bodies, verification of the products where the product’s serialized number can be cross checked with other data to ensure it’s an authentic product. Effective serialization is absolutely essential to avoid traceability complications. Serialization requirements also need to take into account:
- The generation and format of the serialized numbers
- Aggregation of goods that can allow quick and easy scanning of products with their ‘parent vs child’ bundles.
- The guidelines to follow for packaging levels (primary, secondary and tertiary).
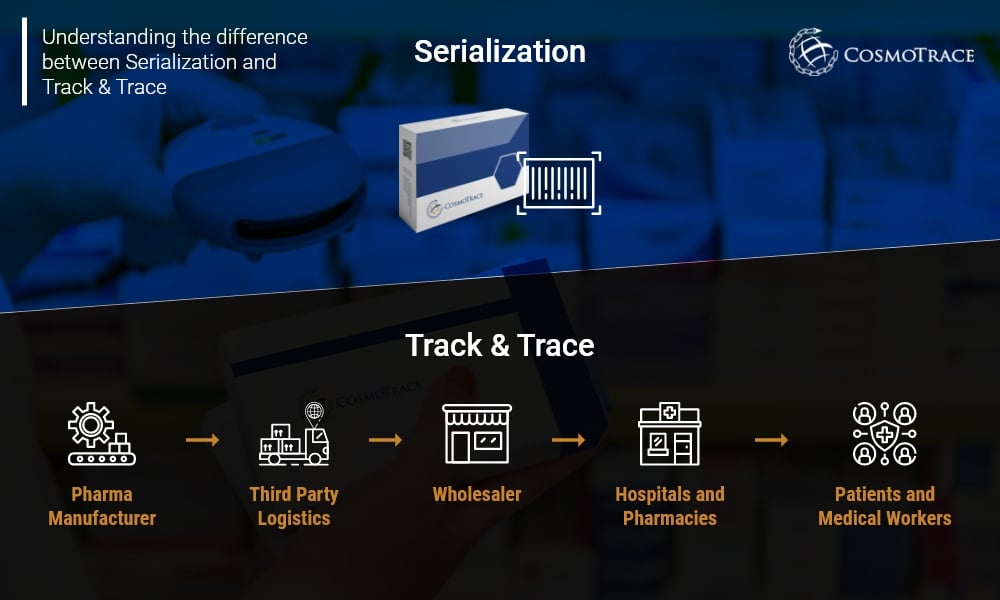
To summarize the difference between Serialization and Track & Trace:
Serialization is when a unique serial number is assigned to each saleable unit of a product which can help in identifying the information of a product’s origin, batch number and expiration date.
Track & Trace in pharma is the process of the product’s current and past locations. It tracks the journey of the product within the supply chain and traces all the intermediate stops it takes from manufacturing to the end user.
The way forward
Advancements in the supply chain industry have created multiple opportunities for the pharma industry as well. With the end goal being optimization and enhancement of the repetitive tasks, businesses are also trying to use to their advantage the power of automation and demanding more insight from their data which is now possible to collect with advancements in areas such as data science.
About CosmoTrace
CosmoTrace was formed by an expert, experienced team that has since worked extensively with every part of the pharmaceutical supply chain from manufacturing through to final distribution. As Serialization Consulting Experts, our focus is on delivering the absolute best serialization support by offering long-term serialization and sustainability strategies and providing forward-thinking industry solutions.
We offer premium support and services that can help your business with serialization implementation. Our team of experts ensure that you are up to date with all global traceability regulations and will help in creating a successful long-term serialization strategy.
To learn more about CosmoTrace visit www.cosmotrace.com. Follow us on LinkedIn and Twitter for Serialization and industry related updates.
Need a helping hand in ensuring smooth operations in your serialization efforts. Contact us to get started.