Gulf Cooperation Council commonly known as GCC was established to harmonize regulations within the GCC countries. One of the key objectives is to help pharmaceutical companies manage drug supply chain traceability. Though each country has its legal guidance for drug traceability the objective is common i.e., to avoid fraudulent drugs getting into the supply chain.
The countries that constitute GCC are Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates (UAE).
Introduction
Gulf Health Council (GHC) is the central drug registration. The agenda for GHC is to make the supply chain safer by building a standardized identification system from drug manufacturing to drug dispensing.
GHC wants drug domestic as well as international manufacturers to adopt GS1 supply-chain standards since GS1 is a widely used trade item identification system worldwide. All drugs’ markings must be upgraded from linear barcodes to GS1 Data Matrix barcodes.

Data Matrix barcode must carry the following data;
- GTIN
- Serial Number
- Batch/Lot Number
- Expiration Date (YYMMDD format)
Aggregation: recommended as per GS1 standards.
Products That Require Barcodes:
- Human products that are packaged and ready to be marketed
- Veterinary products that are packaged and ready to be marketed will require barcoding in the future
Products That Do Not Require Barcodes:
- Free samples of pharmaceutical products
- Nonregistered drugs ordered by hospitals for specific patients and in particular quantities
- Drugs cleared for personal use
- Drugs cleared for repackaging purposes
Implementation Roadmap:
- Phase 1 (MAHs) - December 31, 2021: Serialization and EPCIS reporting to MVC Hub required for Marketing Authorization Holders (MAHs)
- Phase 2 (MAHs) - May 1, 2022: Aggregation to cases and pallets
- Phase 3 (Customs, Agents/Distributors, Pharmacies, Dispensers) - June 1, 2022: Scanning for shipment and medicines authentication
Barcoding requirements:
- Serial Number
- GTIN
- Batch / Lot Number
- Expiry Date
Aggregation: Mandatory
Reporting: NHRA web-portal
Circular No. (05) 2023: To All Agents-Distributors (Importers) - GS1 Participation with use of NHRA-MVC Traceability Hub for Pharmaceutical Product Traceability.
As part of the Traceability initiative, a GS1-provided GLN number is required for all Agents/Distributors (Importers) in the Kingdom who are involved in the following activities:
- Making transactions inside of the DUR and/or OFOQ system relating to imports of pharmaceutical products
- Receiving transactional data from the MAH in the form of EPCIS events
A GLN can be purchased from any authorized GS1 distributor anywhere in the world for some as low as $30 as a one-off fee.
Timelines: 13 December 2022
Serialization – secondary packaging
Data elements: 4 (GS1 format)
- Global Trade Item Number (GTIN)
- Randomized Serial Number (1/10,000 randomization)
- Expiry Date
- Batch or Lot Number
Aggregation: mandatory for manufacturers to aggregate all applicable packaging levels with SSCC
- Trade partners who disaggregate must aggregate with their own SSCC
- Two levels of aggregation hierarchy mandatory for UAE
Products exempted from serialization
- General Sales List (GSL products)
- Medical Devices
- Imported for personal use only
- Free Samples
- Stocks being exported from UAE FTZ
Importation of Unregistered Drugs
When the shipment import permit is created in the MoHAP Import Permit System by “Distributers/ licensed agents/ medical stores”. MoHAP Import Permit System pushes the Unregistered drugs product master data to BrandSync where additional data is entered in BrandSync. BrandSync pushes the master data to Tatmeen.
The arrival of medical products into the UAE FreeTrade Zone territory does not constitute importation until the importation is specifically initiated into the UAE market.
- The Shipping Import Permit contains the product GTIN identifiers for products that are intended to be imported.
- Each product GTIN item on the Shipping Import Permit is valid for a maximum quantity.
The Shipping Import Permit reference must be the same reference specified in each commissioning event in the event list.
The Ministry of Health (MoH) in Kuwait published a Circular in 2022 regarding the track and trace requirements for pharmaceutical medicines. However, as of now, there are no traceability requirements but it is stated that from January 2024 onwards all pharmaceutical products sold in Kuwait must have a GS1 DataMatrix with 4 data elements. Kuwait MoH has planned to implement GS1 standards for serialization, aggregation, and barcoding.
Product Identification
Following 4 data elements to be encoded in 2D barcode. To facilitate the visual correct reading control Human-Readable Information (HRI) is applied along with a 2D barcode:
- GTIN: N2 + N14
- Batch number: N2 + Maximum of 20 alphanumeric characters.
- Expiration date: Represented in YYMMDD.
- Unique Serial number: N2 + up to 20 alphanumeric characters.
Timelines:
1st January-December 2023: Local agents to submit master data to GS1 Kuwait.
1st January 2024: All pharmaceutical products present in the Kuwait supply chain must comply with GS1 DataMatrix with 4 data elements.
* Technical details on master data submission to GS1 Kuwait are awaited.
Gulf Health Council (GHC) is an active organization in GCC of which Kuwait is an active member. It is expected that GHC will announce to initiation Electronic Patient Information Leaflet “e-PIL” project. The agenda of e-PIL is to provide updated, electronic, and harmonized information about medicines throughout the GCC region.
Timelines: complete implementation by 2022 including aggregation by 2019.
Participants: Manufacturer, Distributor, Hospitals/Dispensers, Pharmacies.
Prerequisite: Manufacturer
- Registration: Company, Stakeholder, and Product (Manufactured in KSA and Imported into KSA).
- Reporting of serialization events.
Prerequisite: Distributor
- Registration: Company, Stakeholder, and Product (Imported into KSA).
- Reporting core transactions related to distribution use cases.
Scope of Regulation:
Serialization – secondary (saleable unit) and tertiary (case) level
Data elements; 4 (GS1 format)
- Product Code / GTIN - 14
- Serial Number
- Batch or Lot Number
- Expiry Date
Aggregation: requires aggregation information on secondary packaging, cases, and pallets
- Secondary to Case/Shipper to Pallet
- SFDA defines six packaging levels:
- Primary Level 1 – innermost layer (blister pack or ampule). Not a ‘saleable unit’
- Secondary Level 2 – individual saleable unit. The smallest unit in the aggregation hierarchy.
- Level 3 and Level 4: Homogeneous Bundle and Sub-Carton/Inner Pack.
- Level 5: Case/Carton/Outer Pack
- Level 6: Pallet
- Products Included:
- Prescription Drug Product
- OTC
- Veterinary Pharmaceutical Product
-
Track & Trace Events:
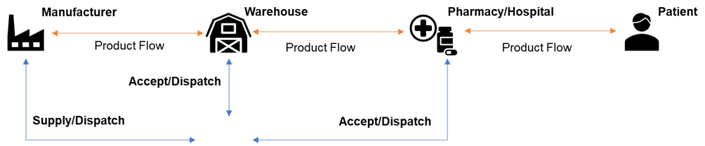
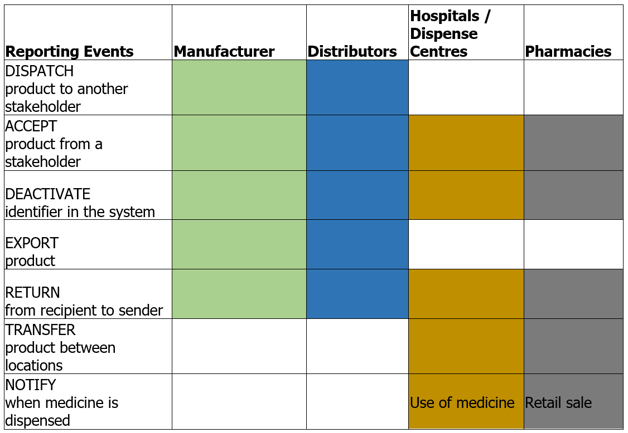
-
SFDA Package Transfer System (PTS)
- Channel for bulk transfer of aggregated product identifiers between trade entities in the supply chain.
- Not a reporting system.
- SFDA has not defined a data format standard.
How can CosmoTrace help?
We provide serialization consulting, implementation, and integration services to help our clients manage end-to-end serialization projects and prepare them for the existing and upcoming regulations across the globe.
Disclaimer
This information is being provided ‘As Is’ with no claims of suitability for a particular purpose. It represents just one possible interpretation of information available in the public domain or through membership organizations, and that interpretation is subject to change. This information does not constitute legal advice. Users must refer to the source material for the complete requirements and form their interpretation before making business decisions. Please use the references below to follow the updates at the source.


