


Serialization Project Management
Sep 16, 2022 | Serialization, Pharmaceutical
With an increasing complexity in the pharmaceutical serialization regulation and supply chain requirements across the globe, project management has become...
NHRA Bahrain Serialization Guideline for Pharma Companies
Apr 8, 2022 | Serialization, Pharmaceutical, Regulations, Compliance
Key dates in Bahrain
Medical Devices Regulations (MDR)
Nov 19, 2021 | Serialization, Pharmaceutical, Regulations, Compliance, Aggregation
Introduction
A complete guide to Brazil Serialization
Nov 8, 2021 | Serialization, Pharmaceutical, Regulations, Compliance, Counterfeit
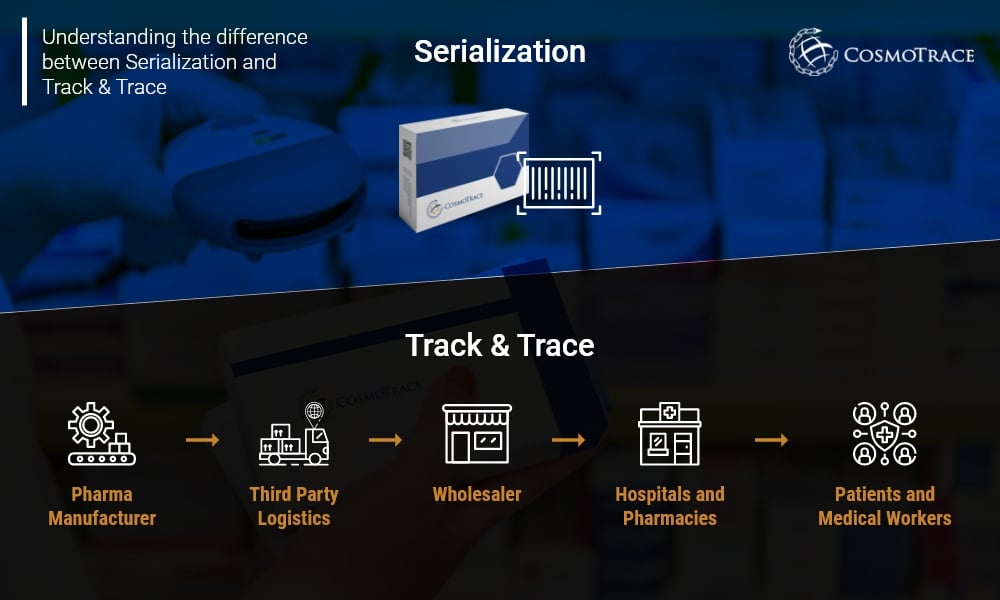
Understanding the difference between Serialization and Track & Trace
Oct 1, 2021 | Serialization, Pharmaceutical
Defining the basics
The need for automation
Sep 24, 2021 | Serialization, Pharmaceutical
Automation has revolutionized manufacturing by boosting production levels to meet increased demand in all industries.
SFDA Dossier requirements
Aug 27, 2021 | Serialization, Pharmaceutical, Regulations, Compliance

The trending supply chain technologies in 2021
Jul 30, 2021 | Serialization, Pharmaceutical, Regulations, Blockchain
The past decade has seen major breakthroughs in technology and though there have been really great strides, the pace of innovation in technology is still...
What is VRS?
Jul 16, 2021 | Serialization, Pharmaceutical
VRS or Verification Router Service is an automated way of verifying saleable returns to improve supply chain efficiency while maintaining the integrity of...